What happens when you bring together over 120 scientists, data experts, and regulatory minds – some gathered in a conference room in Barcelona, others dialling in from around the world? You get two days filled with progress, open debate, and a shared determination to make Virtual Control Groups (VCGs) a reality.
At its core, VICT3R is tackling a complex challenge: replacing concurrent control groups in animal studies with virtual ones, built from carefully curated historical data. It’s a concept with the potential to significantly reduce the number of animals used in toxicology testing – but that’s only possible if regulators have full confidence in the process and the data behind it.
That’s why regulatory acceptance took centre stage at the 2nd VICT3R Consortium Meeting and General Assembly, held on 27-28 February 2025. In just six months, the project has already made impressive progress, including an ongoing scientific advice process with EMA and setting up both an independent Scientific and Regulatory Advisory Board and a dedicated Regulatory Interaction Task Force. These early steps are crucial – no matter how innovative the science is, regulatory buy-in will ultimately determine whether VCGs become part of routine practice.
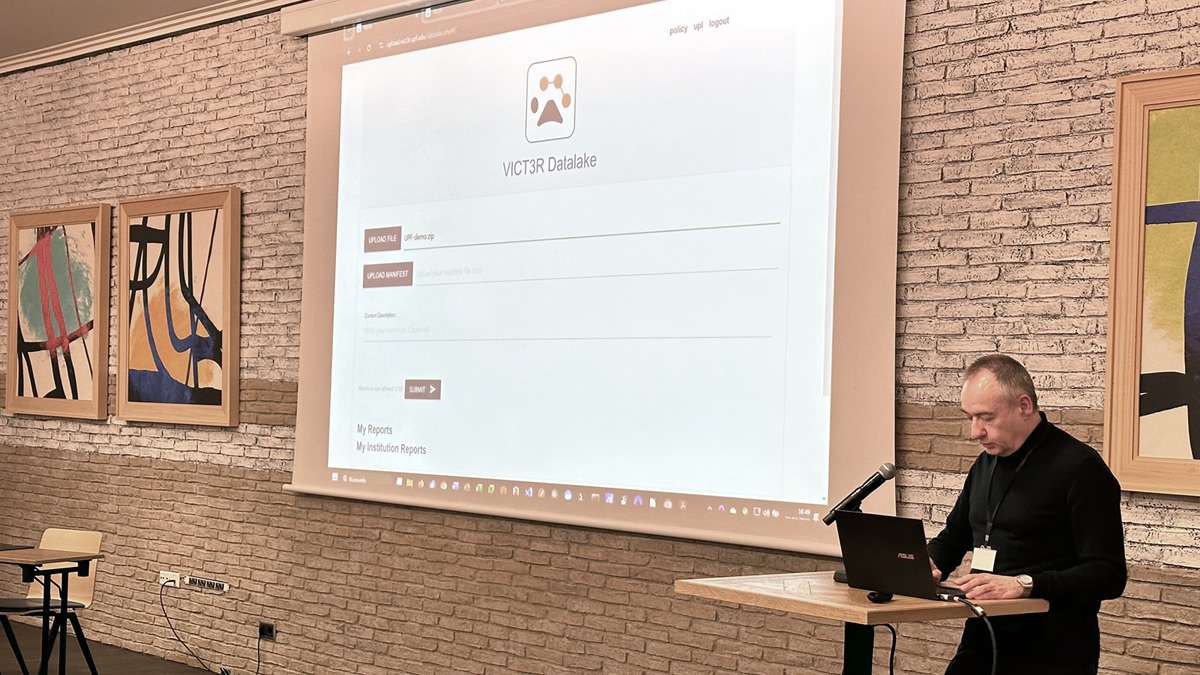
The meeting was also a chance to share progress across all work packages. Partners presented updates on data collection and curation, exploratory data analysis, and provided live demos of the project’s data platform and datalake. There were in-depth discussions around some of the biggest scientific and technical challenges, including the critical question: how do we determine when historical control data is similar enough to concurrent controls in order to be used in a VCG? This is a key issue the consortium continues to explore, with advanced statistical methods and AI tools under development to support this process.
One clear takeaway from the discussions was the importance of keeping scientific, technical, and regulatory workstreams tightly connected. It´s not just a question of generating data – every step needs to be transparent, traceable, and aligned with regulatory expectations. To ensure this, VICT3R’s computer system validation (CSV) process is already up and running, making sure that all systems and processes meet the quality standards regulators will expect.
The meeting wrapped up with valuable feedback from the Scientific and Regulatory Advisory Board, whose members – experts in regulatory science and toxicology – provided both encouragement and clear guidance on priorities for the next phases of the project.
With strong momentum, active engagement across the consortium, and productive dialogue with regulators, VICT3R is well on its way to making Virtual Control Groups a trusted tool – not just for this project, but for the wider scientific and regulatory community. By demonstrating that high-quality science and reducing animal use can go hand in hand, VICT3R is helping shape the future of safety testing.
A big thank you to all the VICT3R community members who contributed to the success of this event. We look forward to welcoming everyone back to Barcelona in September 2025 for the 3rd Consortium Meeting!